Case Study: Suspected Surra in a Horse from Nepalgunj, Banke District, Nepal
Introduction
A 6-year-old male working horse working at a brick kiln in Nepalgunj presented with clinical signs related with trypanosomiasis. The horse’s owner reported a history of recurring fever every 4 to 5 days, accompanied by progressive muscle wasting and lethargy. Given the endemic nature of surra in Nepalgunj and the presence of risk factors such as fly vectors and the labor-intensive use of horses, this case warranted immediate attention.
Clinical Presentation
- Rectal Temperature: 100.1°F
- Pulse: 48-50 per minute
- Respiratory Rate (RR): 12 per minute
Physical Examination Findings
- Emaciated (Progressive weight loss and muscle wasting as reported by farmer)
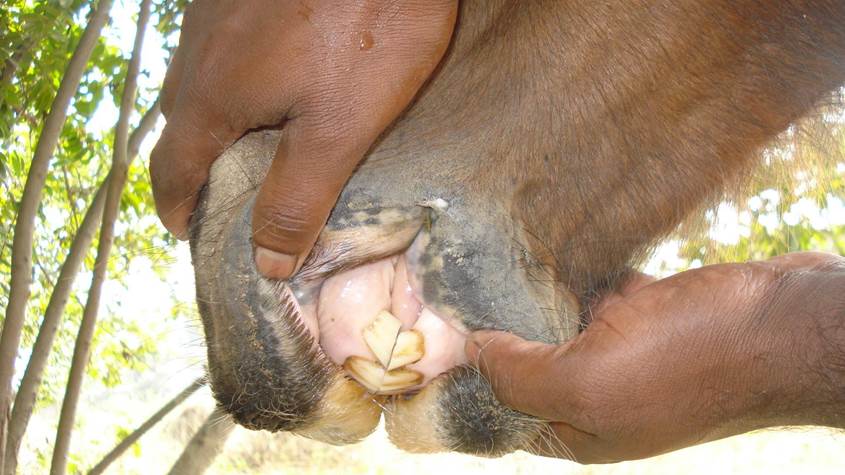
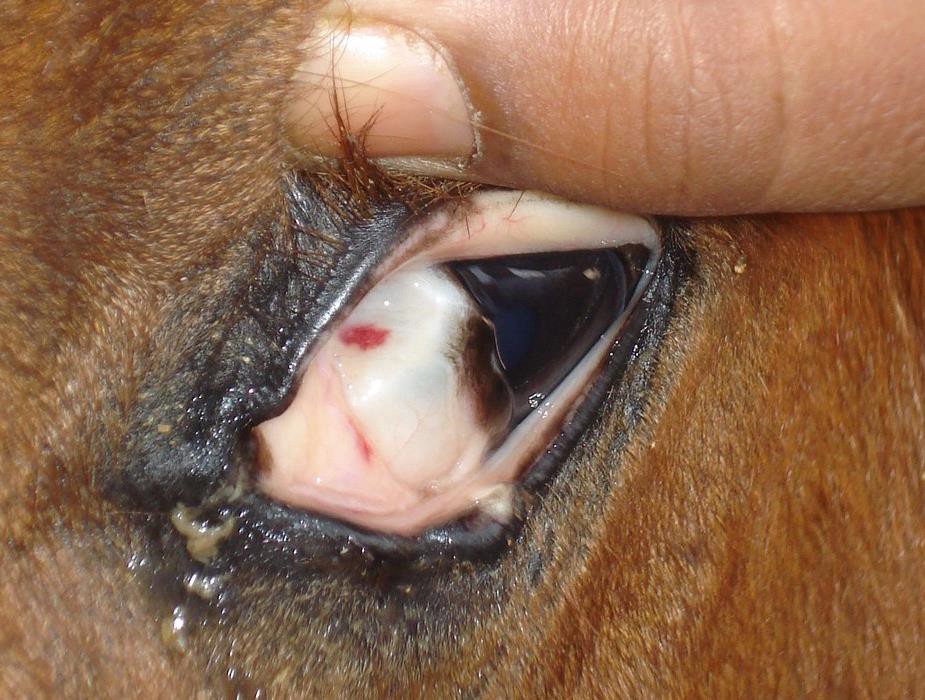
- Petechial hemorrhages on the mucous membranes of the eyes,
- Watery nasal and ocular secretions,
- Depressed and frequent eye closure, and
- Neurological signs, including an abnormal gait
As the horse exhibited signs of fever, lethargy, and neurological impairment, with the additional presence of petechial hemorrhages and ocular discharge. Progressive muscle wasting, neurological signs, and intermittent fever were particularly concerning, as they indicated potentially severe case of Trypanosoma evansi infection.
Diagnosis
The tentative diagnosis was based on the evidence of previous cases of surra in Nepalgunj and on the clinical signs and physical examination. Although parasitemia was not detected immediately, the intermittent nature of parasitemia in trypanosomiasis was considered. A definitive diagnosis would require repeated blood smears or molecular diagnostic tests. Due to the high prevalence of surra in the region, the provisional diagnosis was highly probable.
Treatment
- Phenylbutazone: 5 mg/kg IV q24h for 3 days – to reduce inflammation and alleviate pain.
- Chlortetracycline (CTC): 66 mg/kg BW IV q24h for 5 days – to manage secondary bacterial infections and stabilize systemic health.
- Vitamin B Complex: 10 mL IV q24h for 5 days – to support overall health and counteract potential deficiencies.
- Quinapyramine Sulphate: 3.5 mg/kg BW SC administered after 5 days of CTC treatment – to target the T. evansi infection effectively. For a horse weighing approximately 150 kg, the dose was calculated to be 525 mg.
The treatment protocol was designed to address both the immediate symptoms and underlying infection. Initially, phenylbutazone was administered to reduce systemic inflammation, while chlortetracycline was used to manage secondary bacterial infections, which are common in debilitated animals. Quinapyramine sulphate was scheduled after a five-day interval, which corresponds with the parasite’s life cycle and aims to target circulating parasites when parasitemia is likely to recur.
Supportive Care
- Rest: The horse was allowed a two-week period of rest to minimize physical stress.
- Fly Control: The owner was instructed to implement measures to control fly populations, including the use of fly repellents and maintaining a clean, dry stable environment.
- Muscle Massage: Regular massage was recommended to alleviate muscle stiffness and improve circulation during recovery.
Outcome
After completing the treatment protocol, the horse showed significant improvement. The owner was advised to continue fly control measures and to monitor the animal for any signs of recurrence, particularly during the fly season.
Discussion
Background
Trypanosoma evansi infections in equines, particularly in endemic areas like Nepalgunj, present significant challenges due to the intermittent nature of parasitemia, Progressive wasting, and the potential for neurological involvement. The parasitic infection is transmitted through the bites of infected hematophagous flies, which are prevalent in the region. The case study highlights the importance of timely and appropriate treatment, including anti-inflammatory therapy, antibiotics, and antiprotozoal drugs such as quinapyramine sulphate.
Epidemiology in Nepal
In Nepal, T. evansi infections have been widely reported in areas where equines are used for labor, such as in the Terai region (Singh & Singh, 2016). The prevalence of surra is particularly high in districts like Banke, where the combination of a dense population of flies and the large number of working horses creates an ideal environment for the disease to spread. In such regions, surra is a serious concern for the local equine population, leading to productivity loss and increased veterinary costs (Singh & Singh, 2016). Surveillance in such endemic areas is crucial for early detection and management of the disease.
Prevention in Endemic Regions
- Fly Control Measures: Regular use of insecticides, fly traps, and protective coverings for horses, especially during peak fly activity times.
- Stable Management: Keeping horses in fly-proof stables and ensuring that the stable environment is clean and dry to discourage fly breeding.
- Prophylactic Treatment: Routine administration of quinapyramine sulphate during high-risk periods can help reduce the risk of infection.
- Public Awareness: Educating horse owners on recognizing the signs of surra, the importance of early treatment, and the need for fly control measures.
By addressing these factors, the incidence of surra can be reduced significantly, improving the overall health of the equine population in these endemic areas.
Conclusion
This case study underscores the importance of early detection, effective treatment, and preventive measures in managing Trypanosoma evansi infections in horses. In endemic regions like Nepalgunj, where environmental factors and vector populations facilitate the spread of the disease, comprehensive control strategies, including fly management, veterinary surveillance, and farmer education, are essential. Further research into the biology of the parasite, as well as the development of more effective control measures, will be crucial for mitigating the impact of surra in Nepal’s equine population.
References
Claes, F., Buscher, P., & Touratier, L. (2005). Intermittent parasitemia in Trypanosoma evansi infections. Veterinary Parasitology, 132(3-4), 231-241. https://doi.org/10.1016/j.vetpar.2005.05.030
Moti, Y., Fikru, R., & Delespaux, V. (2012). Epidemiology and diagnosis of animal trypanosomiasis in Ethiopia. Parasites & Vectors, 5(1), 95. https://doi.org/10.1186/1756-3305-5-95
Radostits, O. M., Gay, C. C., Hinchcliff, K. W., & Constable, P. D. (2007). Veterinary Medicine: A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats. Saunders Elsevier.
Singh, A., & Singh, B. (2016). Trypanosomiasis in animals: Challenges and control strategies. Veterinary Parasitology, 229, 71-78. https://doi.org/10.1016/j.vetpar.2016.10.004
Wikipedia. (2024). Trypanosoma evansi. Retrieved from https://en.wikipedia.org/wiki/Trypanosoma_evansi